The Service School: Innovation in Software!
You may be familiar with The Service School, which has been one of Single Source's offerings for years. In the spring of 2017, we really jumped into...
2 min read
Single Source Systems, Inc. : May 26, 2025

For nearly 40 years, Single Source Systems has been helping field service companies in a wide variety of industries, including the Medical Device industry. This industry is one of the most complex, often requiring software applications to be tailored to fit the unique requirements.
Equipment and machinery in this industry can involve advanced imaging capabilities, robotics, nuclear medicine, Artificial Intelligence (AI)-driven interfaces, precision calibration, and regulation mandates. The equipment tends to be very important to the diagnosis and treatment of patients. Labs, clinics, and hospitals can’t afford to have these mission-critical pieces of equipment down for extended periods, jeopardizing patient welfare, the smooth operation of the facility, and necessary cash flow.

This is why service agreements, preventive maintenance, and effective field service are so important to his industry. Lives may depend on it.
“This industry involves highly intricate and complex machinery. Managing field service requires many unique nuances, from the ability to track as-serviced history to the detailed tracking of parent-child relationships of parts,” says Marty Rhodes, president of Single Source Systems. “Because we have worked with many companies in this industry, we have become very familiar with the ins and outs. We speak the language and understand the priorities.”
This familiarity with the industry means Single Source can be highly efficient during implementations, upgrades, programming, or consulting. A company unfamiliar with the industry would require a learning curve that could slow down response time.
“Because we can skip the extensive fact-finding that comes with learning an industry, we can go straight to providing meaningful advice and best practices,” adds Rhodes. “Each time we work with a customer, we learn more about the practical applications of solutions and refine our best practices for that industry. We expand our team's knowledge base. We apply those learned insights to other projects.”
Unit tracking is one example of a specialized need for companies servicing medical devices. Rather than simply tracking by unit, the company may opt to track a multiple-level configuration made up of many “parent” parts that are then made up of “child” parts. This can be important in servicing medical devices. So, adapting software configurations to manage this level of tracking is necessary.
Another unique requirement is regulatory reporting. With 21 CFR Part 11, the Code of Federal Regulations (CFR) establishes the rules for electronic records and signatures set by the United States Food and Drug Administration (FDA) required for medical devices. The code mandates how electronic records are created, modified, maintained, archived, retrieved, or transmitted. Certain service providers must comply with these requirements.

Understanding these specialized needs helps Single Source Systems personalize the service software and configure applications to accommodate these needs.
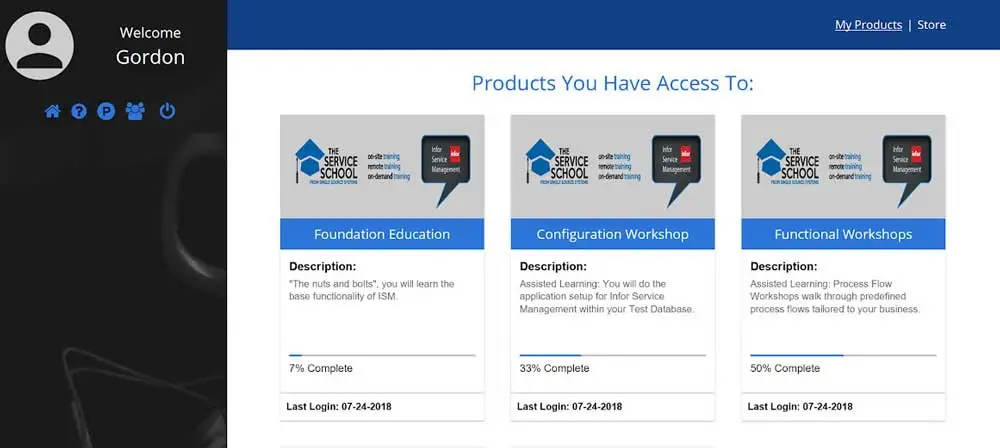
You may be familiar with The Service School, which has been one of Single Source's offerings for years. In the spring of 2017, we really jumped into...
Infor software has become a hot topic throughout the enterprise application solutions market, receiving praise from various industry outlets. Ventana...

The relationship between IT functionality and revenue has never been closer than it is today.